Our Research
Chemical Sustainability
Part of our work focusses on meeting the global challenge of sustainability. Our approach is twofold: lowering the material and energy intensity of chemical reactions, and tapping sustainable or waste sources of synthons. Enzymes, or biocatalysts, have provided a means to address both of these, as they typically operate in benign conditions with high efficiency and selectivity. Taken together, our work on sustainability has four key threads:
Enzyme Discovery
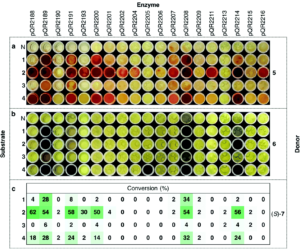
Through metagenomic databases, both public and UCL-developed, we have identified numerous enzymes with industrially-relevant activities. For some of these, colourimetric screening has enabled high-throughput identification of the most efficient homologues, as well as mutagenesis to improve the enzymes beyond their natural capabilities. To date, our suite of biocatalysts include transaminases, transketolases, ene reductases, decarboxylases, tyrosinases, imine reductases, alcohol dehydrogenases, methyltransferases and norcoclaurine synthases.
Biocatalytic Pathways
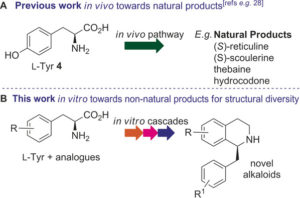
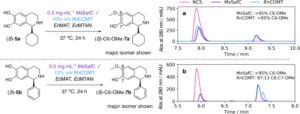
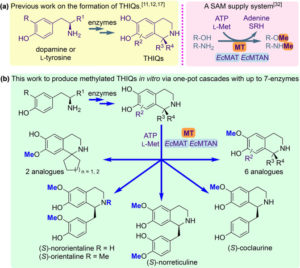
Once discovered and characterised, our enzymes can be linked into multi-step cascades to produce complex molecules from simple starting materials, all in aqueous conditions at low temperatures with minimal side-products. The substrate scope of these biocatalysts, which may be naturally wide or engineered, allows the pathways to be remarkably combinatorial. Whole series of distinct compounds have been generated by feeding slightly different starting materials into the same enzyme ‘cascade’, including biologically active compounds such as protoberberine.
Upgrading waste and plastics
Plastic, specifically PLA and PET, and biomass waste both present potential sources of useful compounds. We are developing enzymes to break down the constituent polymers of these waste products, and upgrade the monomers to useful compounds. This will allow us to both reduce the pollution caused by these waste and develop renewable sources of previously petrochemical-dervied synthons.
Organic synthesis in benign conditions
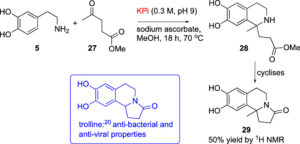
Traditional organic synthesis is largely reliant on harmful solvents, high temperatures and/or pressures and toxic reagents. We are developing synthetic methods which perform well in safer and less environmentally compromising conditions, with the aim to replace their classical counterparts. Tetrahydroisoquinolines have been a particular area of success, for which we have found a phosphate buffer-mediated strategy that allows production of a diverse range of analogues.
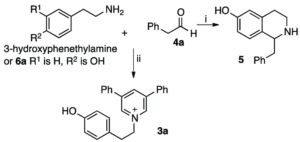
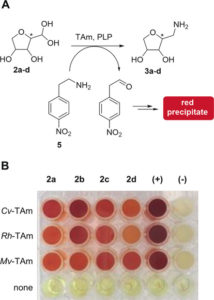
Sketch by Ruby Wright
Chemical Biology
A second branch of our work revolves around using chemistry to directly address biological problems, specifically in a medical context. This has given rise to two distinct avenues of research.
Lipid conjugates for nanoparticles
Alongside collaborators, we have been investigating the structural requirements of the lipids that make up a range of nanoparticles, including ternary systems composed of lipid (L), a targeting peptide (P) and D- or RNA (D or R). We have designed and synthesised several series of novel lipids, which stabilise LPD lipopolyplex particles and enhance transfection efficacy with low toxicity and immunogenicity. In our most recent work, we have synthesised a series of metal-chelating lipid conjugates for in vivo imaging applications.
Novel antimicrobials
We have synthesised and investigated new antimicrobials with novel modes of action. These include tetrahydroisoquinolines and carbazole-based compounds inspired by carprofen, both of which have shown anti-mycobacterial properties when screened by collaborators. Previously, we have investigated the synthesis and biological activity of calixarenes and small molecule analogues with anti-tubercular activities, which appear to act via a host macrophage-mediated mechanism. We have also identified a new route to 1,3,5-N-alkyl pyridiniums using phosphate-based media and, with a collaborator, established that they have antibacterial properties.